Our current focus of studies is towards investigating the cellular level biophysical mechanisms in the bladder that give rise to different facets of bladder function such as bladder contractility, sensory transduction and control of bladder function via known neural pathways. Any deviation from the physiology that underlies bladder function results in urinary incontinence. Urinary incontinence or loss of bladder control is a pathophysiological condition that has its basis in the altered cellular physiology of the components that comprise the bladder wall resulting in symptoms which include bladder overactivity as well as increased neural input to the central nervous system via the afferent pathways. The underlying causes of urinary incontinence are varied and severely impact the quality of life both physiologically as well as psychologically.
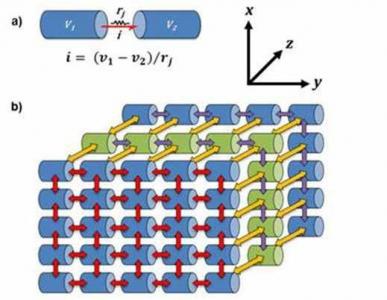
The aim of our research is to study the bladder biophysics in order to demystify the bladder function at cellular and tissue level by developing biophysically detailed computational models that mimic different aspects of bladder activity. The larger aim of our work is to then use our know how to study possible mechanisms that underlie bladder pathophysiology. The composite bladder wall consists of three primary layers, namely, the detrusor smooth muscle, the lamina propria and the bladder epithelia (urothelium). These tissue layers are further composed of structurally and functionally unique components such as smooth muscle cells (SMC), afferent nerve endings (A Delta and C fibers), myofibroblasts, interstitial cells of Cajal (ICC), epithelial cells (umbrella cells) etc. These individual components form networks based on their regions of localisations and there exists pathways via which they can interact with each other, structurally and functionally.
Our current modeling approach includes constructing models for these individual components by endowing them with as many experimentally established mechanisms as possible so as to render them rich in biophysical detail. Typical cellular level mechanisms that describe the functioning and the underlying electrical activity of the bladder wall components include ion channels (voltage, mechanical, temperature, ligand gated), pumps, gap junctions, intracellular calcium dynamics etc. Further, once a sufficiently detailed model for individual components is constructed, our approach then extends to determining their behavior in a network of cells by constructing appropriate models for the same. Finally, our aim is to integrate the various models for the sub components outlined above to create a master model akin to a virtual laboratory that would mimic different aspects of bladder function giving insights into its physiology as well as pathophysiology.
The modeling work being carried out in the lab is broadly subdivided into five domains, namely, (i) modeling of the smooth muscle cells in a syncytial network; (ii) modeling of ionic mechanisms in SMC and intracellular calcium dynamics; (iii) modeling of the reflex pathways involved in bladder control (afferent and efferent); (iv) modeling of sensory transduction pathways in bladder epithelia and lamina propria (ICCs); (v) Classification of the action potentials in SMCs using signal processing techniques. Work carried out in the above domains further branches out into specific areas of focus that is divided between 9 PhD students currently pursuing their doctoral studies at the Computational Neurophysiology lab.
Prof. R Manchanda
